Abstract
Introduction Myeloproliferative neoplasms (MPN) with blast phase (BP) transformation (MPN-BP) are associated with shortened survival since most patients are elderly and unfit for intensive chemotherapy. Venetoclax (Ven) plus hypomethylating agent (HMA) is FDA approved for elderly/unfit acute myeloid leukemia, however MPN-BP patients were excluded from Ven+HMA clinical trials. Accordingly, in the current study, our main objective was to examine Ven+HMA treatment outcomes including the impact of karyotype and mutations on response and survival in MPN-BP.
Methods Patients with MPN-BP that received Ven+HMA outside clinical trials at the Mayo Clinic between July 2018 and May 2022 were retrospectively recruited after institutional review board approval. Diagnosis of MPN-BP required the presence of ≥20% blasts in either the peripheral blood or bone marrow. Cytogenetic and molecular studies were performed by conventional karyotype, and next-generation sequencing, respectively. All patients received at least one cycle of Ven+HMA which included either azacitidine 75 mg/m2 days 1-7 or decitabine 20 mg/m2 days 1-5. Response was assessed according to the 2017 European Leukemia Net (ELN) criteria.
ResultsPatient characteristics 47 patients with MPN-BP (median age 71 years, range 46-84; 60% males) received Ven+HMA either upfront (n=32) or following relapse (n=15). Antecedent MPN included ET/post-ET MF in 18 (38%), PV/post-PV MF in 16 (34%), and PMF in 13 (28%) patients. Driver mutation profile included JAK2 in 76% of the patients and CALR in 18%; other mutations included TP53 in 17 patients (39%), TET2 in 10 (23%), ASXL1 in 15 (34%), IDH1/2 in 12 (27%), EZH2 in 6 (14%), RUNX1 in 6 (14%), N/KRAS,SRSF2 and U2AF1 in 5 (11%) each. ELN cytogenetic risk was favorable (2%), intermediate (34%) or adverse (64%); among the latter, 55% were classified as complex.
Response Thirty-one (66%) patients received decitabine and the remainder azacitidine with median Ven dose of 200 mg for a median of 3 cycles (range, 1-9 cycles). 21 (45%) patients experienced cycle delays/interruptions and treatment was complicated by neutropenic fever in 22 (47%) cases.
CR or CRi was documented in 20 (43%) patients; 12 (26%) patients with CR and 8 (17%) with CRi, and residual morphological features of MPN were present in 10 patients. Among complete responders, median time to response was 1.7 months (range; 1-7 months), with median response duration of 5 months (range, 0.4-35 months). Relapse was documented in 9 (45%) of responding patients. Importantly, 7 of 13 (54%) transplant-eligible patients in CR/CRi, were bridged to transplant.
CR/CRi rates were similar with upfront therapy or in the relapsed setting (47% vs 33%; p=0.38), azacitidine or decitabine (50% vs 39%; p=0.46), presence or absence of TP53 (41% vs 44%; p=0.83), ASXL1 (47% vs 41%, p=0.74), IDH1/2 (50% vs 41%; p=0.58), and K/NRAS mutations (20% vs 46%; p=0.25). CR/CRi was superior without vs with antecedent PV (55% vs 19%, p=0.01), presence vs absence of TET2 mutation (70% vs 35%, p=0.05), and absence of complex karyotype (60%, vs 29%, p=0.04). Multivariable analysis confirmed the favorable impact of TET2 mutation (p=0.02), and absence of antecedent PV (p=0.009) on CR/CRi. Moreover, CR/CRi was significantly higher in TET2 mutated vs unmutated patients without antecedent PV (83% vs 48%) and with antecedent PV (50% vs 9%) (p=0.01).
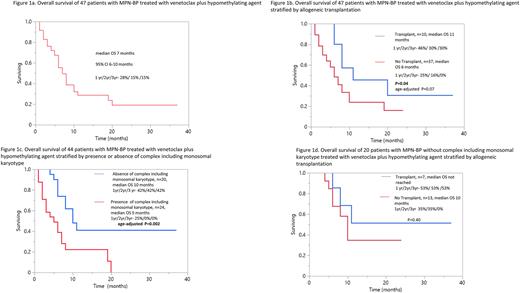
Survival After a median follow up of 6 months (range, 1-37 months) from initiation of Ven+HMA, 31 (66%) patients have died. Median overall survival was 7 months (range; 1-37 months) and longer in transplanted patients (11 vs 6 months; p=0.04) (Figure). On univariate analysis, survival was superior in the absence of complex karyotype (10 vs 5 months, p=0.003), N/KRAS mutations (8 vs 4 months; p=0.02), with achievement of CR/CRi (10 vs 6 months; p=0.02), and transplant (11 vs 6 months, p=0.04). Multivariable analysis confirmed the favorable prognostic impact of absence of complex karyotype and N/KRAS mutations (p=0.003 and 0.03) (Figure).
Conclusions The current study identifies MPN-BP patients with a higher likelihood of response (TET2 mutated without antecedent PV) and prolonged survival (absence of complex karyotype and N/RAS mutations) following treatment with Ven+HMA.
Disclosures
Begna:ImmunoGen: Research Funding; Novartis: Honoraria. Al-Kali:Astex: Other: research support to institution. Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Mangaonkar:Bristol Myers Squibb: Research Funding. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Foran:Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities; AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding. Palmer:Protagonist: Consultancy; Sierra Oncology: Consultancy; CTI BioPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal